Next: All-atom Potential Energy Functions Up: Long-Range Interactions: The Ewald Previous: Ewald Forces
We will consider an Ewald implementation which is a modified version
of the ewald code written for Berend Smit's
Molecular Simulation course. (All of Prof. Smit's codes are available
in the FrenkelSmitCodes directory of the instructional-codes respository.)
This code
simply computes the Ewald energy for a cubic lattice, given an
appropriate number of particles, and a value for
![]() (which is called
(which is called ![]() in the code), and a value for
in the code), and a value for
![]() ,
the maximum integer index for enumerating
,
the maximum integer index for enumerating ![]() -vectors. 1
-vectors. 1
The units used in a system with electrostatics differ depending on
community. So far, we have assumed that the units of electrostatic
potential are charge
![]() , divided by length
, divided by length
![]() ,
because we write potential as
,
because we write potential as
![]() , where
, where ![]() is
measured in units of
is
measured in units of
![]() and distance in units of
and distance in units of
![]() . Energy is therefore written in units of
. Energy is therefore written in units of
![]() over
over
![]() , and force in units of
, and force in units of
![]() over
over
![]() . If we want the final energy in
more familiar units, we can choose
. If we want the final energy in
more familiar units, we can choose
![]() and
and
![]() ,
and use the standard prefactor
,
and use the standard prefactor
![]() to convert from
“charge squared per length” to “energy”. For example, in SI
units,
to convert from
“charge squared per length” to “energy”. For example, in SI
units,
![]() (C
(C![]() /m)/J. In this
implementation, we use a length of
/m)/J. In this
implementation, we use a length of
![]() and
and
![]() and measure energy such that
and measure energy such that
![]() .
.
We will examine two configurations, both with ![]() = 8
= 8![]() = 512
particles, with alternating + and - charges. One configuration has
the particle on a cubic lattice with lattice spacing
= 512
particles, with alternating + and - charges. One configuration has
the particle on a cubic lattice with lattice spacing ![]() , which
is the standard NaCl crystal structure. We will call this the
“crystal” configuration. The other is like the crystal, only each
particle is displaced by a random amount from its Self Part lattice position
with a maximum displacement of 0.3. We will call this the “liquid”
configuration. We compute the total electrostatic energy via the
Ewald sum technique for various values of
, which
is the standard NaCl crystal structure. We will call this the
“crystal” configuration. The other is like the crystal, only each
particle is displaced by a random amount from its Self Part lattice position
with a maximum displacement of 0.3. We will call this the “liquid”
configuration. We compute the total electrostatic energy via the
Ewald sum technique for various values of
![]() and maximum
and maximum
![]() -vector index. As we increase the number of
-vector index. As we increase the number of ![]() -vectors taken in
the sum, we would like to show that the total energy converges to a
certain value. We will measure this in terms of the Madelung
constant,
-vectors taken in
the sum, we would like to show that the total energy converges to a
certain value. We will measure this in terms of the Madelung
constant, ![]() :
:
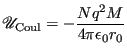 |
(257) |
Table 2 shows results of Ewald summation for the perfect lattice, and Table 3 shows resuts for the “liquid”. We see several interesting things from these example calculations:
|
cfa22@drexel.edu